Abstract
Background
Peripheral T-cell lymphomas (PTCLs) are a rare, aggressive type of non-Hodgkin lymphoma, with frontline treatment (tx) commonly consisting of anthracycline-based, multi-agent chemotherapy regimens, such as cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). Although about half of treatment-naïve patients (pts) achieve a complete remission (CR) (39-55%) (Reimer 2009, d'Amore 2012), long-term survival rates remain low (30-40%) (Ellin 2014). Prior studies of the CD30-targeted antibody-drug conjugate brentuximab vedotin (BV; ADCETRIS®) in relapsed, CD30-expressing PTCLs have shown significant antitumor activity in angioimmunoblastic T-cell lymphoma (AITL), PTCL not otherwise specified (PTCL-NOS) (Horwitz 2014), and systemic anaplastic large cell lymphoma (ALCL). In particular, a pivotal phase 2 study of single-agent BV in pts with relapsed/refractory systemic ALCL resulted in an estimated 5-year overall survival (OS) rate of 60% and progression-free survival (PFS) rate of 39% (Pro 2016), which demonstrates long-term disease control and that a subset of these pts may be potentially cured. This phase 1 study assessed the safety, efficacy, and durability of frontline BV administered in sequence with CHOP, or in combination with CHP (CHOP without vincristine) in pts with CD30-expressing PTCLs (NCT01309789). As previously reported, BV+CHP induced remission in all pts (100% objective response rate), with a CR rate of 88% (Fanale 2014). The end-of-study results are reported here with 5-year survival data and peripheral neuropathy (PN) resolution after BV+CHP.
Methods
Twenty-six adults with untreated, CD30-expressing PTCLs were treated with 1.8 mg/kg BV and standard-dose CHP q3wk for up to 6 cycles. Pts with a CR or partial response could continue on single-agent BV 1.8 mg/kg q3wk for up to 10 additional cycles. Pts who did not have a disease diagnosis of ALCL were required to have ≥1% CD30 expression in malignant cells. Antitumor response was assessed by the investigator per the Revised Response Criteria for Malignant Lymphoma (Cheson 2007) and scans were done at Cycles 6, 12, and 16, and at end of tx. Long-term follow-up (LTFU) visits to evaluate disease status and survival were every 3 months. CT scans were required if progression was suspected. Symptom improvement/resolution of PN was also followed.
Results
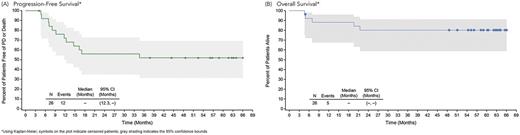
The estimated 5-year PFS and OS rates for all pts who received BV+CHP combination tx were 52% (95% CI: 31, 69) and 80% (95% CI: 59, 91), respectively. No pts underwent a stem cell transplant as consolidation of first remission. At study closure, neither the median PFS (Figure 1A) nor the median OS (Figure 1B) had been reached. Additionally, most ALCL pts were alive (3/3 ALK-positive, 100%; 12/16 ALK-negative, 75%) as well as nearly all non-ALCL pts (6/7, 86%: 2 each with PTCL-NOS, AITL, or adult T-cell leukemia/lymphoma). Four pts with ALK-negative ALCL and 1 with enteropathy-associated T-cell lymphoma died. Four deaths were disease-related, and 1 ALK-negative ALCL pt died due to respiratory failure (disease-relatedness unknown).
After a median observation period of 59.6 months (range, 4.6 to 66.0) from first dose, 14 of 26 pts (54%) remained in remission without any additional therapy. Five pts (19%) with ALK-negative ALCL received retreatment with single-agent BV as a tx for progression in LTFU (prior study tx: 2 pts BV+CHP only; 3 pts BV+CHP with subsequent single-agent BV). Four of these 5 pts were alive at study closure. Three pts received stem cell transplants (1 autologous, 2 allogeneic) for relapsed disease.
Nineteen of 26 pts (73%) had treatment-emergent PN during the study. Of these 19 pts, nearly all (18/19, 95%) experienced either complete resolution (9/19, 47%), or some resolution or improvement (defined as a decrease by at least 1 grade from worst grade; 9/19, 47%). For those with ongoing PN at study closure (10/19, 53%), 1 had Grade 2 PN and 9 had Grade 1 PN. The median time to resolution of PN symptoms was 4.2 months and the median time to improvement of PN symptoms was 2.6 months.
Conclusions
These 5-year durability results appear to demonstrate that initial therapy with BV+CHP induces long-term remissions and may improve cure rates in newly diagnosed CD30-expressing PTCLs. High rates of PN resolution or improvement were also observed. A phase 3 randomized trial comparing BV+CHP with CHOP for the frontline tx of CD30-expressing PTCL is ongoing (NCT01777152).
Fanale: MOLECULAR TEMPLATES: Research Funding; ONYX: Research Funding; GENENTECH: Research Funding; SEATTLE GENETICS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CELGENE: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; TAKEDA: Honoraria, Research Funding; AMGEN: Membership on an entity's Board of Directors or advisory committees; ADC THERAPEUTICS: Research Funding; MERCK: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Horwitz: Infinity/Verastem: Consultancy, Research Funding; BMS: Consultancy; Kyowa-Hakka-Kirin: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Forty-Seven: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Millenium/Takeda: Consultancy, Research Funding; Mundipharma: Consultancy; ADCT Therapeutics: Research Funding; Aileron Therapeutics: Research Funding; HUYA: Consultancy. Forero-Torres: Seattle Genetics (Inst): Research Funding; Immunomedics (Inst): Research Funding; Gilead Sciences (Inst): Research Funding; Pfizer (Inst): Research Funding; Genentech/Roche (Inst): Research Funding; Oncothyreon (Inst): Research Funding; Novartis (Inst): Research Funding; Daiichi Sankyo (Inst): Research Funding; Seattle Genetics: Speakers Bureau; Syndax (Inst): Research Funding; TRACON Pharma (Inst): Research Funding. Bartlett: Bristol-Meyers Squibb: Research Funding; Immune Design: Research Funding; Forty Seven: Research Funding; Affimed: Research Funding; Janssen: Research Funding; Pharmacyclics: Research Funding; Millenium: Research Funding; Astra Zeneca: Research Funding; ImaginAB: Research Funding; Novartis: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Merck & Co: Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding. Advani: Pharmacyclics: Consultancy; Sutro: Consultancy; Merck: Research Funding; Millennium: Research Funding; Gilead: Consultancy; Pharmacyclics: Research Funding; Regeneron: Research Funding; Juno Therapeutics: Consultancy; Cell Medica: Research Funding; Celgene: Research Funding; Genentech: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Spectrum: Consultancy; Seattle Genetics: Research Funding; Nanostring: Consultancy; FortySeven: Research Funding; Janssen: Research Funding; Kura: Research Funding; Bayer Healthcare Pharmaceuticals: Research Funding; Agensys: Research Funding; Infinity: Research Funding. Pro: Seattle Genetics: Consultancy, Other: Travel expenses, Research Funding; Takeda: Honoraria, Other: Travel expenses. Chen: Pharmacyclics: Consultancy, Research Funding; Merck: Consultancy, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Research Funding; Pfizer: Consultancy; Affimed: Research Funding; Seattle Genetics: Consultancy, Research Funding, Speakers Bureau; Genentech: Speakers Bureau. Davies: Janssen: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Karyopharma: Honoraria, Research Funding; Acerta Phamra: Research Funding; Bayer: Research Funding; Takeda: Consultancy, Honoraria, Other: Travel expenses to attend conference, Research Funding; Gilead: Consultancy, Honoraria, Other: Travel expenses to attend conference, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Celgene: Honoraria, Other: Advisory Board, travel expenses to attend conference, Research Funding; GSK: Research Funding; CTI: Consultancy, Honoraria, Other: Travel expenses to attend conference; Seattle Genetics: Research Funding. Illidge: Takeda: Consultancy, Honoraria; Seattle Genetics: Consultancy, Research Funding; Nordic Nanovector: Consultancy, Membership on an entity's Board of Directors or advisory committees; University of Manchester: Consultancy, Employment. Uttarwar: Seattle Genetics: Employment, Equity Ownership. Huebner: Takeda Pharmaceuticals Co.: Employment, Equity Ownership. Ren: Seattle Genetics: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal